When a nonmetal atom gains (an) electron(s), what does it become?
1 Answer
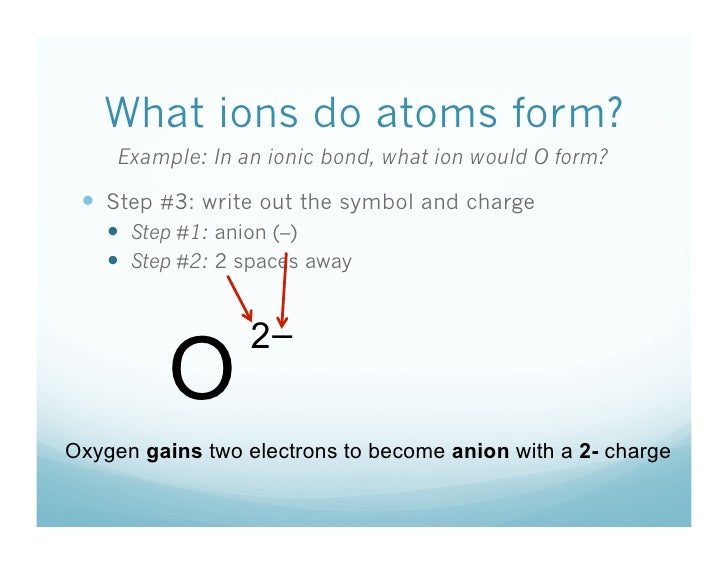
Because electrons are negatively charged, an atom becomes positively or negatively charged as it loses or gains an electron, respectively. Any atom or group of atoms with a net charge (whether positive or negative) is called an ion. A positively charged ion is a cation while a negatively charged ion is an anion. (C) When a neutral atom gains or loses electrons, it becomes an ion An atom becomes an ion with a -1 charge because the atom (A) gains one electron (B) loses one electron.
Explanation:
If An Atom Gains Electrons It Becomes A Part
When an atom gains an electron, it has more electrons (negative) than protons (positive). This means that it has an overall negative charge, and is called an anion (not to be confused with onion).
When An Atom Gains An Electron It Becomes A Positive Ion True Or False
For example, Chlorine
Related questions
