Carbon-14 (146 C) isotope is unstable and radioactive. Carbon-14 decays by emitting beta particles and giving nitrogen. Carbon-14 is an isotope of carbon. It is written as 146 C.
- Relative atomic mass The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted average. Isotopes Atoms of the same element with different numbers of neutrons.
- A neutral atom would have the same number of protons and electrons, so a neutral atom of carbon-12 or carbon-14 would have 6 electrons. Although neutrons do not carry an electrical charge, they have a mass comparable to that of protons, so different isotopes have different atomic weight.
- Z, the atomic number, of carbon is 6. There are 6 massive, positively charged nuclear particles; this gives rise to the elemental identity. If it is the '^14C isotope, there must be 8 neutrally charged massive particles, 8 neutrons, in the nucleus.
- The atomic number and mass number of carbon 14 are, respectively, a) 6 and 8 b) 6 and 14 c) 8 and 14 d) 14 and 20 e) 14 and 22 1 See answer.
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that determines most of the chemical behavior of an element.


In a periodic table arranged in order of increasing atomic number, elements having similar chemical properties naturally line up in the same column (group). For instance, all of the elements in Group 1A are relatively soft metals, react violently with water, and form 1+ charges; all of the elements in Group 8A are unreactive, monatomic gases at room temperature, etc. In other words, there is a periodic repetition of the properties of the chemical elements with increasing mass.

What Is Carbon 14 Atomic Number
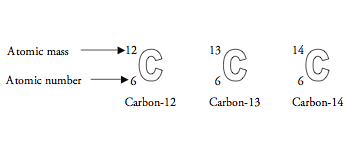
Carbon 14 Atomic Number And Mass Number
In the original periodic table published by Dimitri Mendeleev in 1869, the elements were arranged according to increasing atomic mass— at that time, the nucleus had not yet been discovered, and there was no understanding at all of the interior structure of the atom, so atomic mass was the only guide to use. Once the structure of the nucleus was understood, it became clear that it was the atomic number that governed the properties of the elements.
