- Total Number Of Valence Electrons In Potassium
- Number Of Valence Electrons In Potassium Iodide
- 3 Li - Lithium
Valence Electrons and Ion Formation for the First 20 Elements Element Total Number of Electrons in Neutral Atom Valence Electrons Gain or Lose Electrons Ion Formed Hydrogen 1 1 Gain or Lose 1 H+ or H-Helium 2 2 None None Lithium 3 1 Lose 1 Li+ Beryllium 4 2 Lose 2 Be2+ Boron 5 3 Lose 3 B3+. The total number of electrons present in the valence shell of an atom is called valence electrons, and there is only one electron present in the valence shell of potassium (4s1). Thus, potassium has only one valence electron. Valency of Potassium (K).

What is the number of valence electrons in potassium?
2 Answers
Explanation:
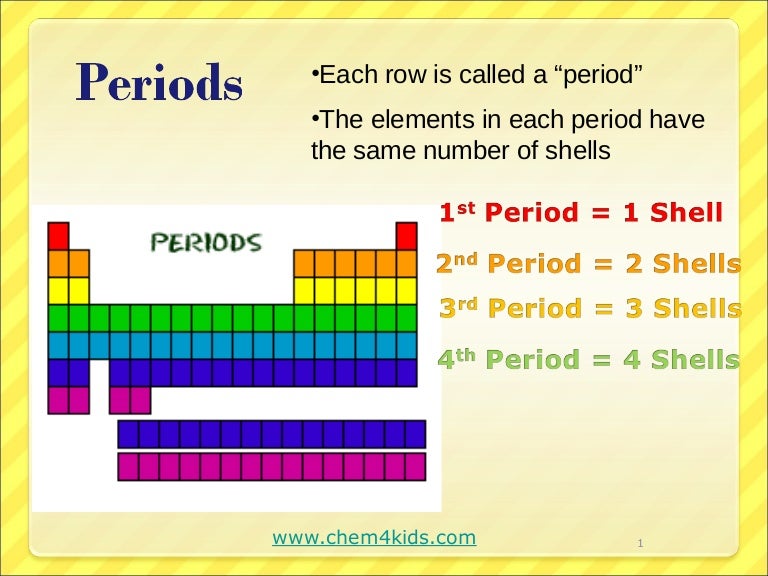
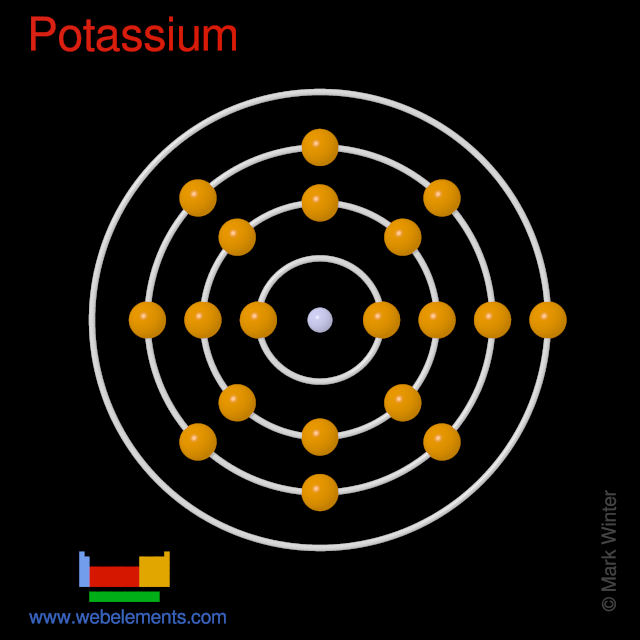
As Potassium is present in the first group of the modern periodic table
Potassium which is also represented as K
it has only ONE valence electron..I.e only on electron is present in the outermost shell
What are valence electrons
Valence electrons are the electrons present in the outermost shell of an atom
Total Number Of Valence Electrons In Potassium
Explanation:
Just like sodium potassium has one valence electron and is highly reactive. (it is monovalent).
Number Of Valence Electrons In Potassium Iodide

3 Li - Lithium
Related questions
